| Methane | |
|---|---|
 | |
| Other names | Marsh gas, firedamp |
| Identifiers | |
| CAS number | 74-82-8 |
| SMILES | |
| InChI | |
| ChemSpider ID | |
| Properties | |
| Molecular formula | CH4 |
| Molar mass | 16.0425 g/mol |
| Appearance | Colorless gas |
| Density | 0.717 kg/m3, gas |
| Melting point | -182.5 °C, 91 K, -297 °F |
| Boiling point | -161.6 °C, 112 K, -259 °F |
| Solubility in water | 3.5 mg/100 mL (17 °C) |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Highly flammable (F+) |
| NFPA 704 | |
| R-phrases | R12 |
| S-phrases | (S2), S9, S16, S33 |
| Flash point | -188 °C |
| Related compounds | |
| Related Alkanes | Ethane, propane |
| Related compounds | Methanol, chloromethane, formic acid, formaldehyde, silane |
| Supplementary data page | |
| Structure and properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references | |
Methane is a chemical compound with the molecular formula CH4. It is the simplest alkane, and the principal component of natural gas. Methane's bond angles are 109.5 degrees. Burning methane in the presence of oxygen produces carbon dioxide and water. The relative abundance of methane and its clean burning process makes it a very attractive fuel. However, because it is a gas at normal temperature and pressure, methane is difficult to transport from its source. In its natural gas form, it is generally transported in bulk by pipeline or LNG carriers; few countries still transport it by truck.
Methane is a relatively potent greenhouse gas with a high global warming potential of 72 (averaged over 20 years) or 25 (averaged over 100 years).[1] Methane in the atmosphere is eventually oxidized, producing carbon dioxide and water. As a result, methane in the atmosphere has a half life of seven years (if no methane were added, then every seven years, the amount of methane would halve). Arctic methane release from melting permafrost and clathrates threatens runaway climate change due to the clathrate gun effect.
The abundance of methane in the Earth's atmosphere in 1998 was 1745 parts per billion, up from 700 ppb in 1750. In the same time period, CO2 increased from 278 to 365 parts per million. The radiative forcing effect due to this increase in methane abundance is about one-third of that of the CO2 increase.[2] In addition, there is a large, but unknown, amount of methane in methane clathrates in the ocean floors. The Earth's crust contains huge amounts of methane. Large amounts of methane are produced anaerobically by methanogenesis. Other sources include mud volcanoes, which are connected with deep geological faults, and livestock, primarily cows.
Contents[hide] |
Properties
Methane is the major component of natural gas, about 87% by volume. At room temperature and standard pressure, methane is a colorless, odorless gas; the smell characteristic of natural gas is an artificial safety measure caused by the addition of an odorant, often methanethiol or ethanethiol. Methane has a boiling point of −161 °C at a pressure of one atmosphere. As a gas it is flammable only over a narrow range of concentrations (5–15%) in air. Liquid methane does not burn unless subjected to high pressure (normally 4–5 atmospheres).
Potential health effects
Methane is not toxic; however, it is highly flammable and may form explosive mixtures with air. Methane is violently reactive with oxidizers, halogens, and some halogen-containing compounds. Methane is also an asphyxiant and may displace oxygen in an enclosed space. Asphyxia may result if the oxygen concentration is reduced to below 19.5% by displacement[citation needed]. The concentrations at which flammable or explosive mixtures form are much lower than the concentration at which asphyxiation risk is significant. When structures are built on or near landfills, methane off-gas can penetrate the buildings' interiors and expose occupants to significant levels of methane. Some buildings have specially engineered recovery systems below their basements to actively capture such fugitive off-gas and vent it away from the building. An example of this type of system is in the Dakin Building, Brisbane, California.
Reactions of methane
Main reactions with methane are: combustion, steam reforming to syngas, and halogenation. In general, methane reactions are hard to control. Partial oxidation to methanol, for example, is difficult to achieve; the reaction typically progresses all the way to carbon dioxide and water.
Combustion
In the combustion of methane, several steps are involved:
Methane is believed to form a formaldehyde (HCHO or H2CO). The formaldehyde gives a formyl radical (HCO), which then forms carbon monoxide (CO). The process is called oxidative pyrolysis:
CH4 + O2 → CO + H2 + H2O
Following oxidative pyrolysis, the H2 oxidizes, forming H2O, replenishing the active species,[clarification needed] and releasing heat. This occurs very quickly, usually in significantly less than a millisecond.
2H2 + O2 →2H2O
Finally, the CO oxidizes, forming CO2 and releasing more heat. This process is generally slower than the other chemical steps, and typically requires a few to several milliseconds to occur.
2CO + O2 →2CO2
The result of the above is the following total equation:
where bracketed "g" stands for gaseous form and bracketed "l" stands for liquid form.
Hydrogen activation
The strength of the carbon-hydrogen covalent bond in methane is among the strongest in all hydrocarbons, and thus its use as a chemical feedstock is limited. Despite the high activation barrier for breaking the C–H bond, CH4 is still the principal starting material for manufacture of hydrogen in steam reforming. The search for catalysts which can facilitate C–H bond activation in methane and other low alkanes is an area of research with considerable industrial significance.
Reactions with halogens
Methane reacts with all halogens given appropriate conditions, as follows:
CH4 + X2 → CH3X + HX
where X is a halogen: fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). This mechanism for this process is called free radical halogenation. When X is Cl, this mechanism has the following form:
- If used isomolecular (equal molecule analogy) quantities in CH2X2, CHX3, even CX4 also produses. Using a large overquantitity of CH4 reduces the production of CH2X2, CHX3, CX4 and more clean CH3X produces.
1. Radical generation:
![Cl_2 \xrightarrow[\triangle]{UV} 2Cl^. - 239 kJ](http://upload.wikimedia.org/math/f/c/7/fc7a8ad8af57852d0e67226ad13a48fa.png)
- The needed energy comes from UV radiation or heating,
2. Radical exchanges:


3. Radiacal extermination:
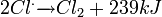


Uses
Fuel
- For more on the use of methane as a fuel, see: natural gas
Methane is important for electrical generation by burning it as a fuel in a gas turbine or steam boiler. Compared to other hydrocarbon fuels, burning methane produces less carbon dioxide for each unit of heat released. At about 891 kJ/mol, methane's combustion heat is lower than any other hydrocarbon; but a ratio with the molecular mass (16.0 g/mol) divided by the heat of combustion (891 kJ/mol) shows that methane, being the simplest hydrocarbon, produces more heat per mass unit than other complex hydrocarbons. In many cities, methane is piped into homes for domestic heating and cooking purposes. In this context it is usually known as natural gas, and is considered to have an energy content of 39 megajoules per cubic meter, or 1,000 BTU per standard cubic foot.
Methane in the form of compressed natural gas is used as a vehicle fuel, and is claimed to be more environmentally friendly than fossil fuels such as gasoline/petrol and diesel.[who?]
Research is being conducted by NASA on methane's potential as a rocket fuel. One advantage of methane is that it is abundant in many parts of the solar system and it could potentially be harvested in situ, providing fuel for a return journey.[4]
Industrial uses
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelines distribute large amounts of natural gas, of which methane is the principal component.
In the chemical industry, methane is the feedstock of choice for the production of hydrogen, methanol, acetic acid, and acetic anhydride. When used to produce any of these chemicals, methane is first converted to synthesis gas, a mixture of carbon monoxide and hydrogen, by steam reforming. In this process, methane and steam react on a nickel catalyst at high temperatures (700–1100 °C).
![CH_4 + H_2O \xrightarrow[700-1100^oC]{Ni} CO + 3H_2](http://upload.wikimedia.org/math/c/d/8/cd8f5a0d855b7ea1e51de43ce679c28c.png)
The ratio of carbon monoxide to hydrogen in synthesis gas can then be adjusted via the water gas shift reaction to the appropriate value for the intended purpose.
CO + H2O → CO2 + H2
Less significant methane-derived chemicals include acetylene, prepared by passing methane through an electric arc, and the chloromethanes (chloromethane, dichloromethane, chloroform, and carbon tetrachloride), produced by reacting methane with chlorine gas. However, the use of these chemicals is declining, acetylene as it is replaced by less costly substitutes, and the chloromethanes due to health and environmental concerns.
Sources of methane
Natural gas fields
The major source of methane is extraction from geological deposits known as natural gas fields. It is associated with other hydrocarbon fuels and sometimes accompanied by helium and nitrogen. The gas at shallow levels (low pressure) is formed by anaerobic decay of organic matter and reworked methane from deep under the Earth's surface. In general, sediments buried deeper and at higher temperatures than those which give oil generate natural gas. Methane is also produced in considerable quantities from the decaying organic wastes of solid waste landfills.
Alternative sources
Apart from gas fields an alternative method of obtaining methane is via biogas generated by the fermentation of organic matter including manure, wastewater sludge, municipal solid waste (including landfills), or any other biodegradable feedstock, under anaerobic conditions. Methane hydrates/clathrates (icelike combinations of methane and water on the sea floor, found in vast quantities) are a potential future source of methane. Cattle belch methane accounts for 16% of the world's annual methane emissions to the atmosphere.[5] The livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane".[6] However animals "that put their energies into making gas are less efficient at producing milk and meat". Early research has found a number of medical treatments and dietary adjustments that help limit the production of methane in ruminants.[7][8][9]
Industrially, methane can be created from common atmospheric gases and hydrogen (produced, for example, by electrolysis) through chemical reactions such as the Sabatier process, Fischer-Tropsch process. Coal bed methane extraction is a method for extracting methane from a coal deposit, while enhanced coal bed methane recovery is a method of recovering methane from an unminable coal seam.
Scientific experiments have given variable results in determining whether plants are a source of methane emissions.[10][11][12]
Methane in Earth's atmosphere
Early in the Earth's history—about 3.5 billion years ago—there was 1,000 times as much methane in the atmosphere as there is now. The earliest methane was released into the atmosphere by volcanic activity. During this time, Earth's earliest life appeared. These first, ancient bacteria added to the methane concentration by converting hydrogen and carbon dioxide into methane and water. Oxygen did not become a major part of the atmosphere until photosynthetic organisms evolved later in Earth's history. With no oxygen, methane stayed in the atmosphere longer and at higher concentrations than it does today.
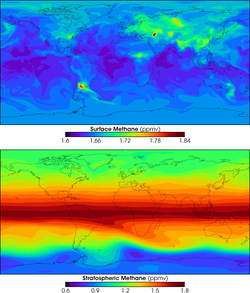
In present times, due to the increase in oxygen, the amount of methane has decreased. The average mole concentration of methane at the Earth's surface in 1998 was 1,745 ppb.[13] Its concentration is higher in the northern hemisphere as most sources (both natural and human) are larger. The concentrations vary seasonally with a minimum in the late summer mainly due to removal by the hydroxyl radical.
Methane is created near the surface, and it is carried into the stratosphere by rising air in the tropics. Uncontrolled build-up of methane in Earth's atmosphere is naturally checked—although human influence can upset this natural regulation—by methane's reaction with hydroxyl radicals formed from singlet oxygen atoms and with water vapor.
Methane as a greenhouse gas
Methane in the Earth's atmosphere is an important greenhouse gas with a global warming potential of 25 over a 100-year period. This means that a methane emission will have 25 times the impact on temperature of a carbon dioxide emission of the same mass over the following 100 years. Methane has a large effect for a brief period (a net lifetime of 8.4 years in the atmosphere), whereas carbon dioxide has a small effect for a long period (over 100 years). Because of this difference in effect and time period, the global warming potential of methane over a 20 year time period is 72. The Earth's methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases.[14] Usually, excess methane from landfills and other natural producers of methane are burned so CO2 is released into the atmosphere instead of methane because methane is such a more effective greenhouse gas.




No comments:
Post a Comment